Determine the concentration of H3O+ to the correct number of significant figures in a solution with each pH. Describe how these calculations show the relationship between the number of digits to the right of the decimal place in pH and the number of significant figures in concentration. pH = 2.50 pH = 2.51 pH = 2.52
Ch.16 - Acids and Bases
Chapter 16, Problem 57c
For each strong acid solution, determine [H3O+], [OH–], and pH. c. a solution that is 0.052 M in HBr and 0.020 M in HNO3
 Verified step by step guidance
Verified step by step guidance1
<strong>Step 1:</strong> Recognize that both HBr and HNO<sub>3</sub> are strong acids, which means they completely dissociate in water. Therefore, the concentration of H<sub>3</sub>O<sup>+</sup> ions from each acid is equal to the initial concentration of the acid itself.
<strong>Step 2:</strong> Calculate the total [H<sub>3</sub>O<sup>+</sup>] by adding the contributions from both acids: [H<sub>3</sub>O<sup>+</sup>] = [HBr] + [HNO<sub>3</sub>].
<strong>Step 3:</strong> Use the ion product of water, K<sub>w</sub> = 1.0 x 10<sup>-14</sup>, to find [OH<sup>-</sup>]. The relationship is given by [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>] = K<sub>w</sub>. Solve for [OH<sup>-</sup>].
<strong>Step 4:</strong> Calculate the pH of the solution using the formula pH = -log[H<sub>3</sub>O<sup>+</sup>].
<strong>Step 5:</strong> Review your calculations to ensure that the assumptions of complete dissociation and the use of K<sub>w</sub> are valid for the given concentrations.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Acids and Ionization
Strong acids, such as HBr and HNO3, completely dissociate in water, meaning that they release all of their hydrogen ions (H+) into the solution. This complete ionization leads to a direct relationship between the concentration of the acid and the concentration of hydronium ions ([H3O+]), which can be calculated from the molarity of the acid.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
pH Calculation
The pH of a solution is a measure of its acidity, defined as the negative logarithm of the hydronium ion concentration: pH = -log[H3O+]. For strong acids, the pH can be easily calculated using the concentration of the acid, as it directly corresponds to the concentration of H3O+ in the solution.
Recommended video:
Guided course

pH Calculation Example
Relationship Between [H3O+] and [OH-]
In aqueous solutions, the product of the concentrations of hydronium ions and hydroxide ions ([H3O+][OH-]) is constant at 25°C, known as the ion product of water (Kw = 1.0 x 10^-14). Therefore, once [H3O+] is determined from the strong acid concentration, [OH-] can be calculated using the equation [OH-] = Kw / [H3O+].
Recommended video:
Guided course
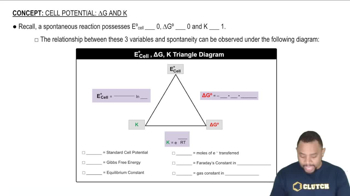
Relationship between ∆E°, ∆G°, and K
Related Practice
Textbook Question
2010
views
Textbook Question
For each strong acid solution, determine [H3O+], [OH–], and pH. a. 0.25 M HCl
714
views
1
rank
Textbook Question
For each strong acid solution, determine [H3O+], [OH–], and pH. b. 0.015 M HNO3
441
views
Textbook Question
For each strong acid solution, determine [H3O+], [OH–], and pH. d. a solution that is 0.655% HNO3 by mass (assume a density of 1.01 g/mL for the solution)
1747
views
Textbook Question
Determine the pH of each solution. a. 0.048 M HI b. 0.0895 M HClO4 c. a solution that is 0.045 M in HClO4 and 0.048 M in HCl d. a solution that is 1.09% HCl by mass (assume a density of 1.01 g/mL for the solution)
Textbook Question
What mass of HI must be present in 0.250 L of solution to obtain a solution with each pH value?
a. pH = 1.25 b. pH = 1.75 c. pH = 2.85
108
views
