Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Acids and Bases
Lewis acids are species that can accept an electron pair, while Lewis bases are species that can donate an electron pair. This definition expands the concept of acids and bases beyond protons (H+) to include electron pair interactions, making it applicable to a wider range of chemical reactions.
Recommended video:
Electron Pair Donation
In the context of Lewis theory, a Lewis base donates an electron pair to form a covalent bond with a Lewis acid. This donation is crucial in understanding how different chemical species interact, as it determines the direction of electron flow and the formation of new compounds.
Recommended video:
Valence Shell Electron Pair Repulsion Theory
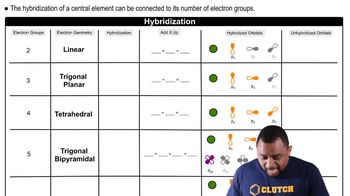
Molecular Geometry and Hybridization
The molecular geometry and hybridization of a species influence its ability to act as a Lewis acid or base. For example, BeCl2 has a linear geometry and does not have a lone pair of electrons, which makes it a Lewis acid as it can accept electron pairs from bases during chemical reactions.
Recommended video:
Hybridization and Electron Geometry

 Verified step by step guidance
Verified step by step guidance