The activation barrier for the hydrolysis of sucrose into glucose and fructose is 108 kJ/mol. If an enzyme increases the rate of the hydrolysis reaction by a factor of 1 million, how much lower must the activation barrier be when sucrose is in the active site of the enzyme? (Assume that the frequency factors for the catalyzed and uncatalyzed reactions are identical and a temperature of 25 °C.)
Ch.14 - Chemical Kinetics
Chapter 14, Problem 83b
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) b. What is the half-life for this reaction (at the initial concentration)?

Verified Solution
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Half-life
Half-life is the time required for the concentration of a reactant to decrease to half of its initial value. It is a crucial concept in kinetics, particularly for first-order reactions, where the half-life is constant and independent of concentration. Understanding half-life allows chemists to predict how long it will take for a reaction to reach a certain point, which is essential for analyzing reaction rates.
Recommended video:
Guided course
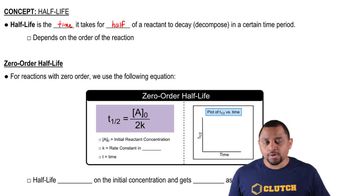
Zero-Order Half-life
Reaction Kinetics
Reaction kinetics is the study of the rates of chemical reactions and the factors that affect these rates. It involves understanding how concentration, temperature, and catalysts influence the speed of a reaction. In this context, knowing the kinetics of the reaction between CH3CN and CH3NC is necessary to calculate the half-life and understand the reaction's behavior over time.
Recommended video:
Guided course

Chemical Kinetics
Concentration and Rate Laws
Concentration refers to the amount of a substance in a given volume, which directly impacts the rate of a chemical reaction. Rate laws express the relationship between the rate of a reaction and the concentration of its reactants. For the given reaction, determining the initial concentration of CH3CN is essential for calculating the half-life, as it influences how quickly the reaction proceeds.
Recommended video:
Guided course

Concentration Changes and Rate Law Example
Related Practice
Textbook Question
1809
views
Open Question
How long will it take for 90% of the CH3CN to convert to CH3NC at 500 °C given the tabulated data: Time (h) [CH3CN] (M) 0.0 1.000, 5.0 0.794, 10.0 0.631, 15.0 0.501, 20.0 0.398, 25.0 0.316?
Textbook Question
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) a. Determine the order of the reaction and the value of the rate constant at this temperature.
1711
views
Open Question
What is the half-life for this reaction at the initial concentration?
Textbook Question
The tabulated data were collected for this reaction at a certain temperature: X2Y → 2 X + Y a. Determine the order of the reaction and the value of the rate constant at this temperature.
756
views
Textbook Question
The tabulated data were collected for this reaction at a certain temperature: X2Y → 2 X + Y c. What is the concentration of X after 10.0 hours?
1122
views
