The decomposition of XY is second order in XY and has a rate constant of 7.02⨉10-3 M-1• s-1 at a certain temperature. a. What is the half-life for this reaction at an initial concentration of 0.100 M?
The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. How long does it take for 25% of the C-14 atoms in a sample of C-14 to decay?

Verified Solution
Key Concepts
Half-life
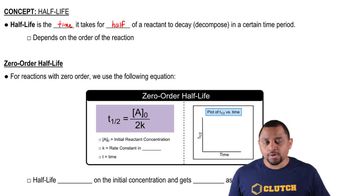
Radioactive Decay

Exponential Decay

The half-life for the radioactive decay of U-238 is 4.5 billion years and is independent of initial concentration. How long will it take for 10% of the U-238 atoms in a sample of U-238 to decay?
The half-life for the radioactive decay of U-238 is 4.5 billion years and is independent of initial concentration. If a sample of U-238 initially contained 1.5⨉1018 atoms when the universe was formed 13.8 billion years ago, how many U-238 atoms does it contain today?
The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. If a sample of C-14 initially contains 1.5 mmol of C-14, how many millimoles are left after 2255 years?
The diagram shows the energy of a reaction as the reaction progresses. Label each blank box in the diagram.
a. reactants b. products c. activation energy (Ea) d. enthalpy of reaction (ΔHrxn)
The activation energy of a reaction is 56.8 kJ/mol and the frequency factor is 1.5⨉1011/ s. Calculate the rate constant of the reaction at 25 °C.
