Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Life
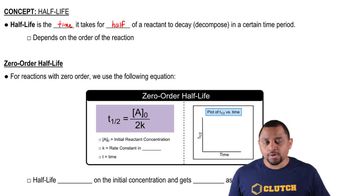
Half-life is the time required for half of the radioactive nuclei in a sample to decay. For first-order reactions, this is a constant value that characterizes the decay rate of the substance. In the case of plutonium-239, its half-life is 24,000 years, meaning after this period, half of the original amount will remain.
Recommended video:
First-Order Kinetics
First-order kinetics refers to a reaction rate that is directly proportional to the concentration of one reactant. In radioactive decay, the rate at which a substance decays is proportional to the amount of the substance present. This means that as the quantity decreases, the rate of decay also decreases, leading to an exponential decay pattern.
Recommended video:
Exponential Decay
Exponential decay describes the process by which a quantity decreases at a rate proportional to its current value. In radioactive decay, the amount of substance remaining can be calculated using the formula N(t) = N0 * e^(-kt), where N0 is the initial quantity, k is the decay constant, and t is time. This concept is crucial for determining how long it takes for a substance to decay to a specific amount, such as one atom.
Recommended video:

 Verified step by step guidance
Verified step by step guidance