Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Order
Reaction order refers to the power to which the concentration of a reactant is raised in the rate law of a chemical reaction. It indicates how the rate of reaction depends on the concentration of reactants. For example, a second-order reaction means that the rate is proportional to the square of the concentration of the reactant, which significantly influences how the concentration changes over time.
Recommended video:
Half-Life in Second-Order Reactions
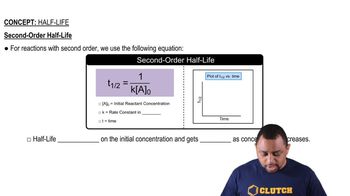
The half-life of a substance in a second-order reaction is inversely proportional to the initial concentration of the reactant. Unlike first-order reactions, where the half-life is constant, the half-life for second-order reactions increases as the reaction proceeds. This means that as time goes on, it takes longer for the concentration of the reactant to decrease by half.
Recommended video:
Integrated Rate Law for Second-Order Reactions
The integrated rate law for a second-order reaction can be expressed as 1/[X] = kt + 1/[X]₀, where [X] is the concentration of the reactant at time t, k is the rate constant, and [X]₀ is the initial concentration. This equation allows us to calculate the concentration of the reactant at any given time, which is essential for determining how much of substance X remains after a specified duration.
Recommended video: