Textbook Question
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
b. Identify the intermediates in the mechanism.

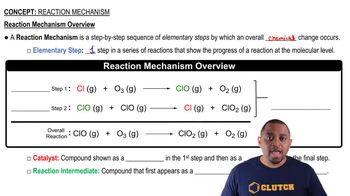


Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
b. Identify the intermediates in the mechanism.
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
c. What is the predicted rate law?
Many heterogeneous catalysts are deposited on high-surfacearea supports. Why?
Suppose that the reaction A¡products is exothermic and has an activation barrier of 75 kJ/mol. Sketch an energy diagram showing the energy of the reaction as a function of the progress of the reaction. Draw a second energy curve showing the effect of a catalyst.