Here are the essential concepts you must grasp in order to answer the question correctly.
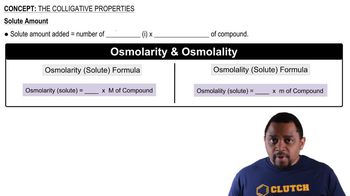
Colligative Properties
Colligative properties are physical properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. Understanding these properties is essential for calculating changes in physical states when solutes are added to solvents.
Recommended video:
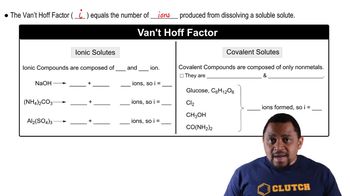
van't Hoff Factor (i)
The van't Hoff factor (i) is a dimensionless number that represents the number of particles a solute dissociates into when dissolved in a solvent. For ionic compounds like iron(III) chloride (FeCl3), which dissociates into four ions (one Fe³⁺ and three Cl⁻), the van't Hoff factor is 4. This factor is crucial for accurately calculating colligative properties, as it directly influences the extent of property changes.
Recommended video:
Freezing Point Depression
Freezing point depression is a colligative property that describes the decrease in the freezing point of a solvent when a solute is added. The extent of freezing point depression can be calculated using the formula ΔTf = i * Kf * m, where ΔTf is the change in freezing point, Kf is the freezing point depression constant of the solvent, and m is the molality of the solution. This concept is vital for determining the new freezing point of a solution containing iron(III) chloride.
Recommended video:
Freezing Point Depression
 Verified step by step guidance
Verified step by step guidance