Here are the essential concepts you must grasp in order to answer the question correctly.
Colligative Properties
Colligative properties are physical properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. In this context, the boiling point elevation of the solution is used to determine the van't Hoff factor.
Recommended video:
Boiling Point Elevation
Boiling point elevation is a colligative property that describes how the boiling point of a solvent increases when a solute is dissolved in it. The change in boiling point can be calculated using the formula ΔT_b = i * K_b * m, where ΔT_b is the boiling point elevation, i is the van't Hoff factor, K_b is the ebullioscopic constant of the solvent, and m is the molality of the solution. This concept is crucial for solving the given problem.
Recommended video:
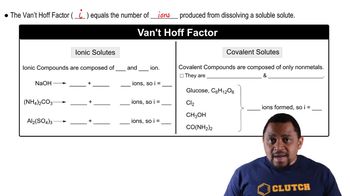
van't Hoff Factor (i)
The van't Hoff factor (i) represents the number of particles into which a solute dissociates in solution. For ionic compounds, this factor is typically greater than one, as they dissociate into multiple ions. For the compound MX2, which dissociates into one M ion and two X ions, the expected van't Hoff factor would be 3. Calculating i involves using the observed boiling point elevation to find the effective number of particles in solution.
Recommended video: