An ibuprofen suspension for infants contains 100 mg/5.0 mL suspension. The recommended dose is 10 mg/kg body weight. How many mL of this suspension should be given to an infant weighing 18 lb? (Assume two significant figures.)
Ch.1 - Matter, Measurement & Problem Solving
Chapter 1, Problem 107
Classify each property as intensive or extensive. a. volume b. boiling point c. temperature d. electrical conductivity e. energy
 Verified step by step guidance
Verified step by step guidance1
Understand the difference between intensive and extensive properties: Intensive properties do not depend on the amount of substance present, while extensive properties do depend on the amount of substance.
Classify volume: Volume is an extensive property because it changes with the amount of substance present.
Classify boiling point: Boiling point is an intensive property because it remains the same regardless of the amount of substance.
Classify temperature: Temperature is an intensive property because it does not depend on the quantity of the substance.
Classify electrical conductivity and energy: Electrical conductivity is an intensive property, while energy is an extensive property because it depends on the amount of substance.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Intensive Properties
Intensive properties are characteristics of a substance that do not depend on the amount of material present. Examples include boiling point, temperature, and electrical conductivity. These properties are intrinsic to the material itself, allowing for identification and classification regardless of sample size.
Recommended video:
Guided course
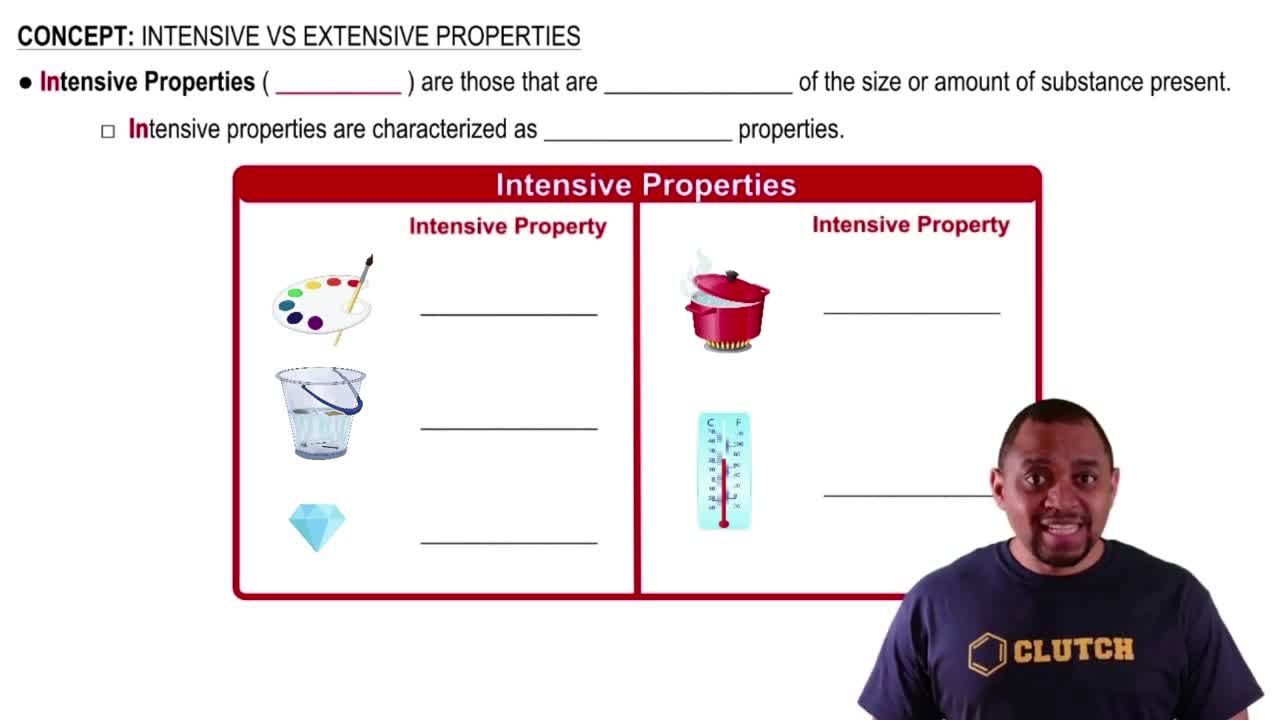
Intensive Properties
Extensive Properties
Extensive properties are characteristics that do depend on the amount of substance present. Volume and energy are prime examples of extensive properties, as they increase with the quantity of material. Understanding the distinction between extensive and intensive properties is crucial for classifying physical properties accurately.
Recommended video:
Guided course
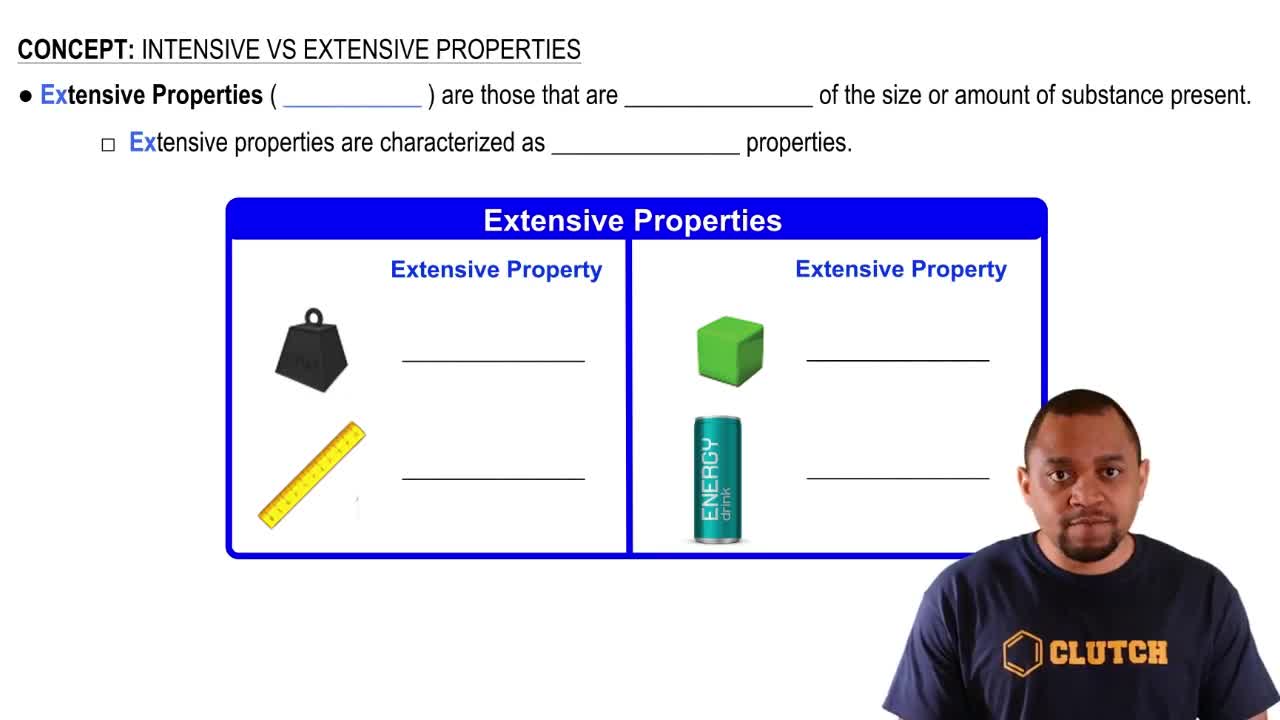
Extensive Properties
Classification of Properties
Classifying properties as intensive or extensive helps in understanding the behavior of substances under various conditions. This classification is fundamental in chemistry, as it aids in predicting how substances will react or change when manipulated, such as during chemical reactions or physical changes.
Recommended video:
Guided course

Element Classification Example
Related Practice
Textbook Question
4073
views
3
rank
Textbook Question
There are exactly 60 seconds in a minute, exactly 60 minutes in an hour, exactly 24 hours in a mean solar day, and 365.24 solar days in a solar year. How many seconds are in a solar year? Give your answer with the correct number of significant figures.
2889
views
Textbook Question
Determine the number of picoseconds in 2.0 hours.
778
views
Textbook Question
At what temperatures are the readings on the Fahrenheit and Celsius thermometers the same?
989
views
Open Question
Suppose you design a new thermometer called the X thermometer. On the X scale, the boiling point of water is 130 °X, and the freezing point of water is 10 °X. At what temperature are the readings on the Fahrenheit and X thermometers the same?
Textbook Question
On a new Jekyll temperature scale, water freezes at 17 °J and boils at 97 °J. On another new temperature scale, the Hyde scale, water freezes at 0 °H and boils at 120 °H. If methyl alcohol boils at 84 °H, what is its boiling point on the Jekyll scale?
846
views
1
rank
