Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when atoms transfer electrons, resulting in the formation of charged ions. These compounds typically consist of a metal and a non-metal, where the metal loses electrons to become a positively charged cation, and the non-metal gains electrons to become a negatively charged anion. Calcium chloride (CaCl2) is an example of an ionic compound, as it consists of calcium ions (Ca²⁺) and chloride ions (Cl⁻) held together by strong electrostatic forces.
Recommended video:
Molecular Solids
Molecular solids are composed of molecules held together by intermolecular forces such as van der Waals forces, hydrogen bonds, or dipole-dipole interactions. These solids typically have lower melting and boiling points compared to ionic or atomic solids due to the weaker forces between the molecules. Examples include ice (solid water) and sugar, which consist of discrete molecules rather than ions or atoms.
Recommended video:
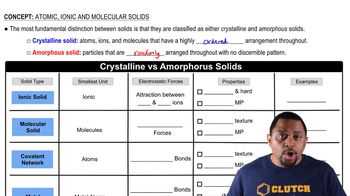
Crystalline vs Amorphous Solids
Atomic Solids
Atomic solids are composed of atoms held together by covalent bonds, forming a network structure. These solids exhibit high melting points and are often very hard due to the strong bonding between atoms. Diamond and silicon are classic examples of atomic solids, where each atom is bonded to several others in a three-dimensional lattice, resulting in their characteristic properties.
Recommended video:
Crystalline vs Amorphous Solids
 Verified step by step guidance
Verified step by step guidance