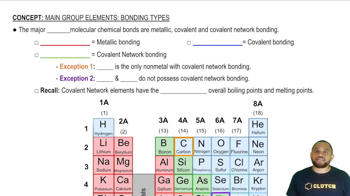
Textbook Question
The following diagrams illustrate p-p orbital overlap or s-p
orbital overlap. Which diagram represents a p bond in valence
bond theory? (LO 8.3)
(a)
(b)
(c)
(d)
656
views
 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 8
Problem 8