Here are the essential concepts you must grasp in order to answer the question correctly.
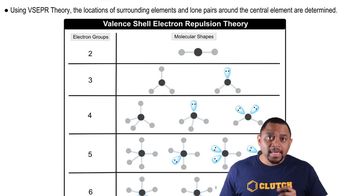
VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of molecules based on the repulsion between electron pairs in the valence shell of atoms. According to this theory, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular shapes. For urea, this model helps in predicting the arrangement of atoms around the central carbon and nitrogen atoms.
Recommended video:
Molecular Shapes and VSEPR
Hybridization
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate the bonding requirements of a molecule. In urea, the carbon atom is typically sp² hybridized, while the nitrogen atom is sp³ hybridized. This hybridization influences the bond angles and the overall geometry of the molecule, which can be analyzed using the VSEPR model.
Recommended video:
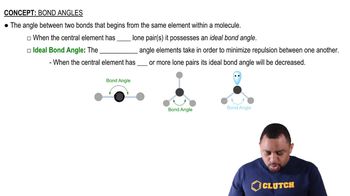
Bond Angles
Bond angles are the angles formed between adjacent bonds in a molecule, which are influenced by the hybridization of the atoms involved. In urea, the expected bond angles based on the VSEPR model would be approximately 120° for the sp² hybridized carbon and 109.5° for the sp³ hybridized nitrogen. However, actual bond angles may vary slightly due to factors such as lone pair repulsion and the presence of different atoms.
Recommended video: