Write the symbol, give the ground-state electron configuration, and draw an orbital-filling diagram for each of the following atoms. Use the abbreviation of the preceding noble gas to represent the inner-shell electrons. (a) The heaviest alkaline earth metal
Ch.5 - Periodicity & Electronic Structure of Atoms
Chapter 5, Problem 111b
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (b) Can have ml = -1

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Quantum Numbers
Quantum numbers are a set of numerical values that describe the unique quantum state of an electron in an atom. The four quantum numbers include the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). Each number provides specific information about the electron's energy level, shape, orientation, and spin.
Recommended video:
Guided course

Principal Quantum Number
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) determines the orientation of an orbital in space and can take on integer values ranging from -l to +l, where l is the azimuthal quantum number. For example, if l = 1 (p subshell), ml can be -1, 0, or +1. This concept is crucial for understanding how orbitals are arranged in a magnetic field and how they interact with other orbitals.
Recommended video:
Guided course
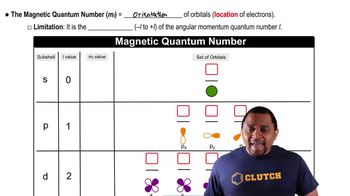
Magnetic Quantum Number
Subshells and Their Designations
Subshells are divisions of electron shells that contain orbitals of the same type. They are designated by the letters s, p, d, and f, corresponding to different values of l (0, 1, 2, and 3, respectively). In this context, the 2p subshell has l = 1, allowing for ml values of -1, 0, and +1, while the 3p subshell also allows ml = -1, making them relevant to the question.
Recommended video:
Guided course

Angular Momentum Quantum Number and Subshell
Related Practice
Textbook Question
727
views
Textbook Question
Write the symbol, give the ground-state electron configuration, and draw an orbital-filling diagram for each of the following atoms. Use the abbreviation of the preceding noble gas to represent the inner-shell electrons.
(c) The heaviest actinide metal
820
views
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (a) Has l = 2
638
views
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (e) Contains the outermost electrons in a beryllium atom
517
views
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (f) Can contain two electrons, both with spin ms = +1/2
666
views
Textbook Question
At what atomic number is the filling of a g orbital likely to begin?
714
views
