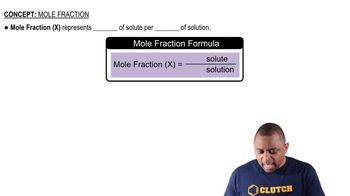
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity (M)
Molarity is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in moles per liter (mol/L). Understanding molarity is crucial for calculating how much solute is needed to achieve a desired concentration in a given volume of solution.
Recommended video:
Moles of Solute
A mole is a unit that quantifies the amount of substance. One mole contains approximately 6.022 x 10^23 entities (Avogadro's number). To find the number of moles needed for a solution, you multiply the molarity by the volume of the solution in liters, which is essential for determining how much solute to weigh out.
Recommended video:
Mass of Solute
The mass of solute can be calculated using the formula: mass = moles × molar mass. The molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). This concept is vital for converting the calculated number of moles into grams, allowing for the accurate preparation of solutions.
Recommended video: