Textbook Question
The following two redox reactions occur between aqueous cations and solid metals. Will a solution of green cations react with solid blue metal? Explain.
(a)
(b)
351
views


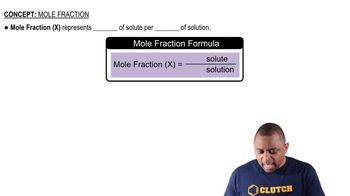

The following two redox reactions occur between aqueous cations and solid metals. Will a solution of green cations react with solid blue metal? Explain.
(a)
(b)
How many moles of solute are present in each of the following solutions? (a) 35.0 mL of 1.200 M HNO3
How many moles of solute are present in each of the following solutions? (b) 175 mL of 0.67 M glucose (C6H12O6)
How many grams of solute would you use to prepare each of the following solutions? (b) 167 mL of 0.200 M boric acid (H3BO3)
How many milliliters of a 0.45 M BaCl2 solution contain 15.0 g of BaCl2?