Textbook Question
Predict the number of unpaired electrons for each of the following.
(a) Sc3+
(b) Co2+
83
views



Predict the number of unpaired electrons for each of the following.
(a) Sc3+
(b) Co2+
What is the general trend in standard potentials for the oxidation of first-series transition metals from Sc to Zn? What is the reason for the trend?
What is the highest oxidation state for each of the elements from Sc to Zn?
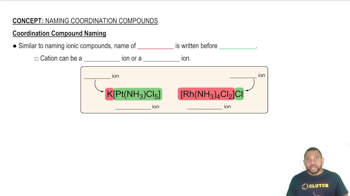
What is the oxidation state of the metal in each of the complexes?
a. AgCl2–
b. [Cr(H2O)5Cl]2+
c. [Co(NCS)4]2–
d. [ZrF8]4–
e. [Fe(EDTA)(H2O)]–
What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
What role does EDTA4- play as a trace additive to mayonnaise? Would the glycinate ion (H2NCH2CH2NH2) be an effective substitute for EDTA4-?