The [Cr(H2O)6]3+ ion is violet, and [Cr(CN)6]3- is yellow. Explain this difference using crystal field theory. Use the colors to order H2O and CN- in the spectrochemical series.
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.108
Problem 21.108The Ni2+(aq) cation is green, but Zn2+(aq) is colorless. Explain.

Verified Solution
Key Concepts
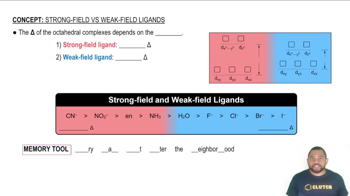
Transition Metal Ions and Color

Electronic Configuration of Ions

Ligand Field Theory
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [CrF6]3-
(b) [V(H2O)6]3+
(c) [Fe(CN)6]3-
Draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons for each of the following.
(a) [Cu(en)3]2+
(b) [FeF6]2-
(c) [Co(en)3]3+ (low spin)
Draw a crystal field energy-level diagram for a square planar complex, and explain why square planar geometry is especially common for d8 complexes.
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(d) [Cu(en)2]2+ (square planar)
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [Pt(NH3)4]2+ (square planar)