 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.102
Problem 21.102Draw a crystal field energy-level diagram for the 3d orbitals of titanium in [Ti(H2O)6]3+]. Indicate the crystal field splitting, and explain why is [Ti(H2O)6]3+] colored.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Crystal Field Theory

Crystal Field Splitting Energy (Δ)
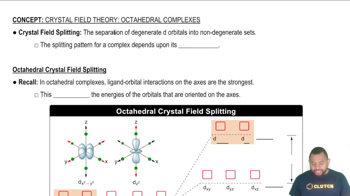
Color in Transition Metal Complexes

What hybrid orbitals are used by the metal ion and how many unpaired electrons are present the complex ion [VCl4]- with tetrahedral geometry?
(a) sp3; 2 unpaired electrons
(b) sp3; 3 unpaired electrons
(c) sp3d2; 3 unpaired electrons
(d) sp3d2; 4 unpaired electrons
What is the electron configuration of Co2+ and how many unpaired electrons are in the free transition metal ion?
(a) [Ar]3d54s2; 5 unpaired electrons
(b) [Ar]3d54s2; 1 unpaired electron
(c) [Ar]3d7; 3 unpaired electrons
(d) [Ar]3d7; 1 unpaired electron
The [Cr(H2O)6]3+ ion is violet, and [Cr(CN)6]3- is yellow. Explain this difference using crystal field theory. Use the colors to order H2O and CN- in the spectrochemical series.
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [CrF6]3-
(b) [V(H2O)6]3+
(c) [Fe(CN)6]3-
Draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons for each of the following.
(a) [Cu(en)3]2+
(b) [FeF6]2-
(c) [Co(en)3]3+ (low spin)