Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Law
Mendeleev's periodic law states that the properties of elements are a periodic function of their atomic weights. This means that when elements are arranged in order of increasing atomic weight, elements with similar properties appear at regular intervals. This foundational principle allowed Mendeleev to organize the periodic table and predict the properties of undiscovered elements based on their position.
Recommended video:
Element Properties and Trends
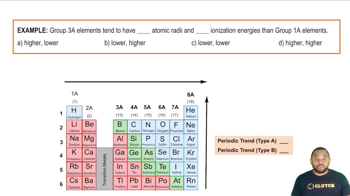
Elements in the periodic table exhibit trends in properties such as electronegativity, atomic radius, and ionization energy. Mendeleev recognized these trends and used them to infer the characteristics of elements that had not yet been discovered. For example, he could predict that elements below aluminum (Al) and silicon (Si) would share similar chemical behaviors due to their placement in the same group.
Recommended video:
Main Group Elements: Periodic Trends Example
Gaps in the Periodic Table
Mendeleev intentionally left gaps in his periodic table for elements that had not yet been discovered, believing that these elements would eventually be found. He predicted their existence and properties based on the patterns he observed in the table. This foresight demonstrated his understanding of the periodic nature of elements and the relationships between them, which later proved to be accurate with the discovery of new elements.
Recommended video:
Periodic Table Classifications