Here are the essential concepts you must grasp in order to answer the question correctly.
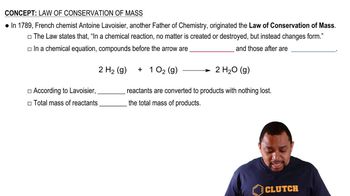
Law of Conservation of Mass
The Law of Conservation of Mass states that mass is neither created nor destroyed in a chemical reaction. This principle implies that the total mass of the reactants must equal the total mass of the products, regardless of whether the reaction is endothermic or exothermic.
Recommended video:
Law of Conservation of Mass
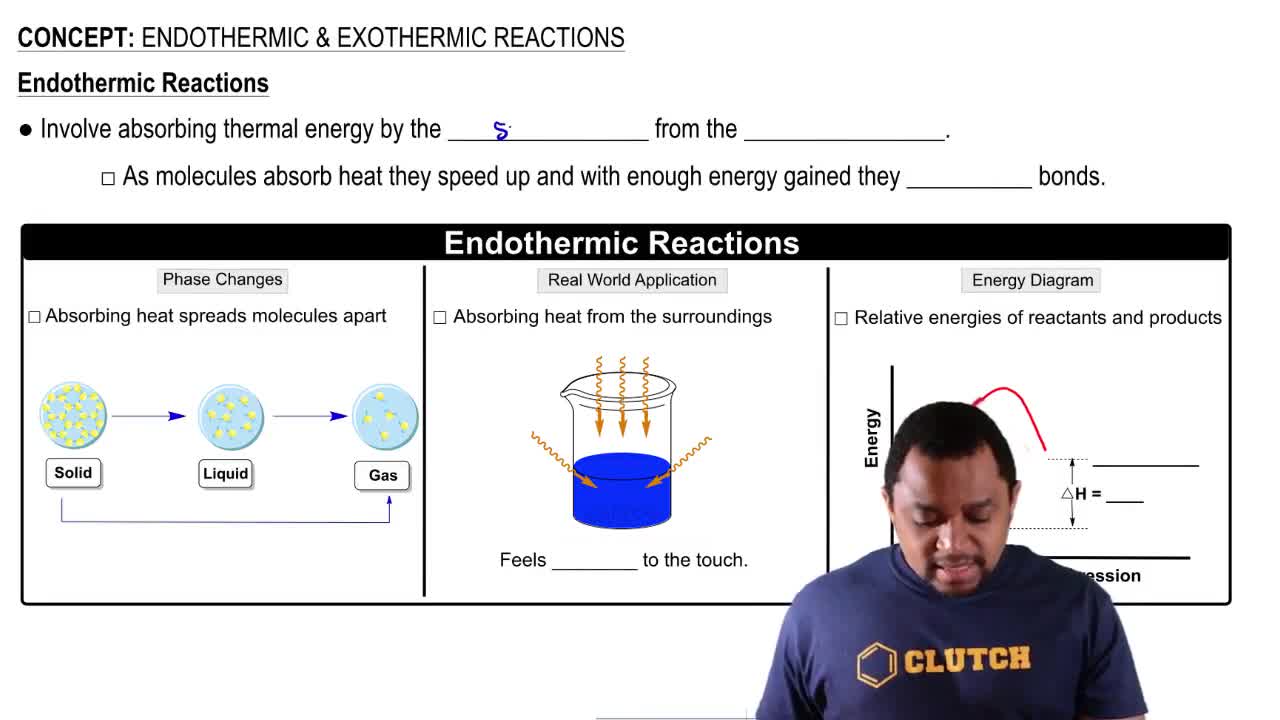
Endothermic Reactions
Endothermic reactions are processes that absorb energy, usually in the form of heat, from their surroundings. This energy absorption can lead to a temperature drop in the environment, but it does not affect the mass of the reactants and products involved in the reaction.
Recommended video:
Endothermic & Exothermic Reactions
Chemical Reaction Products
In a chemical reaction, the products are the substances formed as a result of the reaction between the reactants. In the context of the question, the mass of the products will be equal to the mass of the reactants, as dictated by the Law of Conservation of Mass, regardless of the energy changes involved.
Recommended video:
Production of Hydrogen Example