A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (a) What is the concentration of H3O+ at the first equiva-lence point?

Verified Solution
Key Concepts
Titration and Equivalence Points

Strong vs. Weak Acids
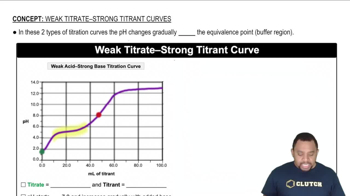
Concentration Calculations

A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (b) What are the initial concentrations of HCl and H3PO4 in the mixture?
A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (c) What percent of the HCl is neutralized at the first equivalence point?
A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (f) What indicators would you select to signal the equiva-lence points?
