Here are the essential concepts you must grasp in order to answer the question correctly.
Band Theory
Band theory explains the electronic structure of solids, particularly metals and semiconductors, by describing how atomic orbitals combine to form energy bands. In transition metals, the presence of partially filled d-orbitals allows for the formation of conduction bands, which influence electrical and thermal properties, including melting points.
Recommended video:
Intepreting the Band of Stability
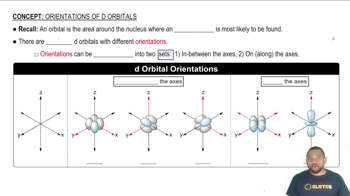
Transition Metals and d-Orbitals
Transition metals are characterized by their d-orbitals, which can accommodate varying numbers of electrons. The filling of these d-orbitals affects the metallic bonding strength and, consequently, the melting points. As the d-orbitals fill, the increased electron-electron interactions can lead to stronger metallic bonds, resulting in higher melting points.
Recommended video:
Melting Point Trends in Transition Metals
The melting points of transition metals exhibit a unique trend due to the interplay of metallic bonding and crystal structure. Initially, melting points increase due to stronger bonding from filled d-orbitals, peaking at elements like molybdenum. However, as the d-orbitals become more filled and the structure changes, the melting points can decrease, as seen in cadmium, where weaker bonding leads to lower melting temperatures.
Recommended video:
Boiling Point and Melting Point