Here are the essential concepts you must grasp in order to answer the question correctly.
Standard State
The standard state of a substance refers to its physical state (solid, liquid, or gas) at a specified temperature and pressure, typically 1 bar (100 kPa) and 25°C (298 K). Understanding the standard state is crucial for determining the phase of elements and compounds under standard conditions, as it provides a reference point for thermodynamic calculations.
Recommended video:
Standard Reduction Potentials
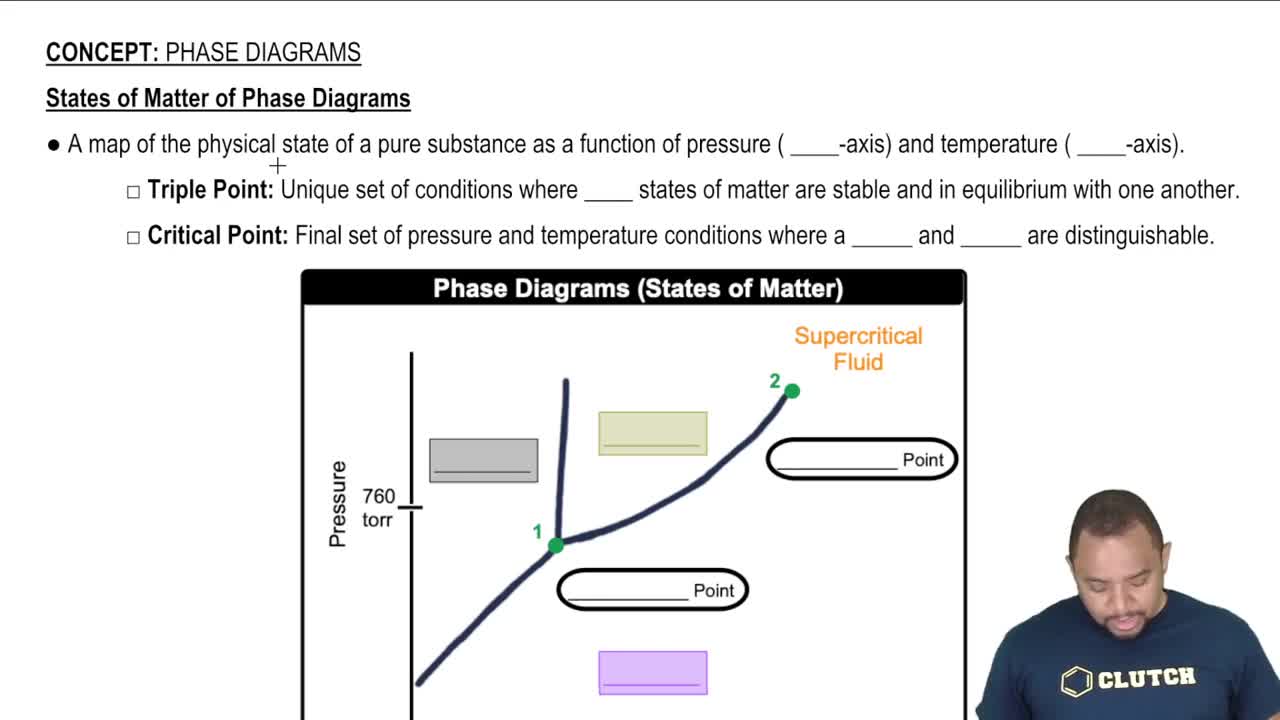
Phase of Matter
The phase of matter describes the distinct forms that different phases of matter take on. The three primary phases are solid, liquid, and gas, each characterized by different properties such as shape, volume, and molecular arrangement. For example, while metals like iron (Fe) are solid at room temperature, gases like nitrogen (N2) exist in a gaseous state.
Recommended video:
States of Matter of Phase Diagrams
Element vs. Compound
Elements are pure substances that consist of only one type of atom, while compounds are substances formed from two or more different types of atoms chemically bonded together. This distinction is important when identifying the phases of substances, as elements like iron (Fe) and nitrogen (N2) exhibit different physical states compared to compounds like ammonia (NH3) and bromine (Br2) under standard conditions.
Recommended video:
Homonuclear vs. Heteronuclear Compounds
 Verified step by step guidance
Verified step by step guidance