What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.78b
Problem 21.78b Verified step by step guidance
Verified step by step guidance

What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
What role does EDTA4- play as a trace additive to mayonnaise? Would the glycinate ion (H2NCH2CH2NH2) be an effective substitute for EDTA4-?
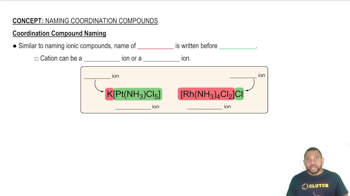
What is the systematic name for each of the following ions?
(a) [MnCl4]2-
(b) [Ni(NH3)6]2+
Assign a systematic name to each of the following ions.
(a) [AuCl4]-
(b) [Fe(CN)6]4-
Assign a systematic name to each of the following ions.
(c) [Fe(H2O)5NCS]2+
(d) [Cr(NH3)2(C2O4)2]-
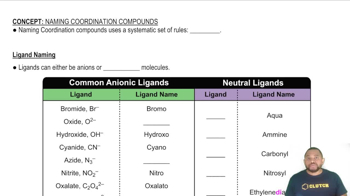
Based on the wavelength of maximum absorption of the cobalt complexes, arrange the following ligands in a spectrochemical series from weakest-field to strongest-field ligand.
(a) Cl- < NCS- < H2O < NH3
(b) Cl- < NCS- < H2O < NH3
(c) H2O < Cl- < NH3 < NCS-
(d) Cl- < H2O < NCS- < NH3