What is the highest oxidation state for each of the elements from Sc to Zn?
Ch.21 - Transition Elements and Coordination Chemistry
Chapter 21, Problem 21.65
What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
 Verified step by step guidance
Verified step by step guidance1
Identify the charge of each ligand in the complex. For example, CN^- has a charge of -1, CO is neutral, en (ethylenediamine) is neutral, H2O is neutral, Br^- has a charge of -1, C2O4^2- (oxalate) has a charge of -2, NH3 is neutral, and NO2^- has a charge of -1.
For each complex, set up an equation where the sum of the oxidation state of the metal and the charges of the ligands equals the overall charge of the complex.
Solve the equation for the oxidation state of the metal. For example, in [Ni(CN)5]3-, the equation would be: x + 5(-1) = -3, where x is the oxidation state of Ni.
Repeat the process for each complex: [Ni(CN)5]3-, Ni(CO)4, [Co(en)2(H2O)Br]2+, [Cu(H2O)2(C2O4)2]2-, and Co(NH3)3(NO2)3.
Verify your results by ensuring that the calculated oxidation state and the charges of the ligands add up to the overall charge of the complex.

Verified Solution
Video duration:
0m:0sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation State
The oxidation state, or oxidation number, is a measure of the degree of oxidation of an atom in a chemical compound. It indicates the number of electrons that an atom can gain, lose, or share when forming chemical bonds. Understanding oxidation states is crucial for determining the charge of metal ions in coordination complexes, which is essential for solving the given question.
Recommended video:
Guided course

Oxidation Numbers
Coordination Complexes
Coordination complexes consist of a central metal atom or ion bonded to surrounding molecules or ions, known as ligands. The nature of these ligands, whether they are neutral or charged, affects the overall charge of the complex and the oxidation state of the metal. Recognizing the types of ligands and their charges is vital for accurately calculating the oxidation states in the provided complexes.
Recommended video:
Guided course
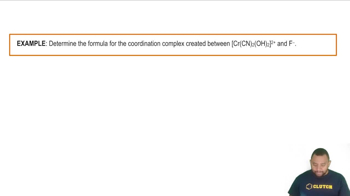
Coordination Complexes Example
Ligand Charge and Coordination Number
The charge of ligands and the coordination number, which is the number of ligand atoms bonded to the central metal, play a significant role in determining the oxidation state of the metal. For example, neutral ligands like CO and NH3 do not contribute to the overall charge, while charged ligands like CN- and NO2- do. Analyzing these factors helps in deducing the oxidation state of the metal in each complex.
Recommended video:
Guided course

Coordination Numbers
Related Practice
Textbook Question
101
views
Textbook Question
What is the name of the compound [Fe(H2O)5(SCN)]Cl2?
(a) pentaaquathiocyanatoiron(III) chloride
(b) pentaaquachlorothiocyanato iron(III)
(c) pentaaquathiocyanatoiron(III) dichloride
(d) pentaaquathiocyanatoiron(II) chloride
491
views
Textbook Question
What is the oxidation state of the metal in each of the complexes?
a. AgCl2–
b. [Cr(H2O)5Cl]2+
c. [Co(NCS)4]2–
d. [ZrF8]4–
e. [Fe(EDTA)(H2O)]–
89
views
Textbook Question
What role does EDTA4- play as a trace additive to mayonnaise? Would the glycinate ion (H2NCH2CH2NH2) be an effective substitute for EDTA4-?
70
views
Textbook Question
What is the systematic name for each of the following ions?
(a) [MnCl4]2-
(b) [Ni(NH3)6]2+
132
views
Textbook Question
What is the systematic name for each of the following ions?
(c) [Co(CO3)3]3-
(d) [Pt(en)2(SCN)2]2+
117
views
