Textbook Question
What is the group number of the green, blue, and red elements?
443
views
 Verified step by step guidance
Verified step by step guidance


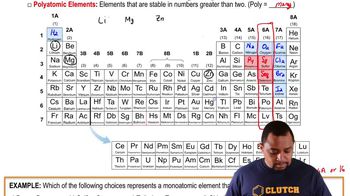
What is the group number of the green, blue, and red elements?
Name at least one other element that is chemically similar to the green element.
Classify these elements as metals, nonmetals, or semimetals.
Would you expect these elements to have similar or different chemical reactivity?
If yellow spheres represent sulfur atoms and red spheres represent oxygen atoms, which of the following drawings shows a collection of sulfur dioxide (SO2) units?
(a)
(b)
(c)
(d)