Here are the essential concepts you must grasp in order to answer the question correctly.
IUPAC Nomenclature
IUPAC nomenclature is a systematic method for naming chemical compounds, ensuring that each name conveys specific information about the compound's structure. It involves rules for naming organic and inorganic compounds, including the identification of functional groups, oxidation states, and coordination numbers. Understanding these rules is essential for accurately naming compounds like lead(IV) acetate.
Recommended video:
Coordination Compounds
Coordination compounds consist of a central metal atom or ion bonded to surrounding molecules or ions called ligands. In the case of Pb(CH₃CO₂)₄, lead is the central metal, and acetate ions act as ligands. Recognizing the nature of these interactions is crucial for determining the correct systematic name and understanding the compound's properties.
Recommended video:
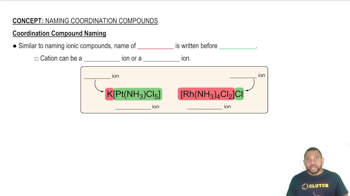
Coordination Compound Naming
Oxidation States
The oxidation state of an element in a compound indicates the degree of oxidation or reduction it has undergone. For lead in Pb(CH₃CO₂)₄, the oxidation state is +4, which is important for naming the compound correctly. Knowing how to determine oxidation states helps in understanding the compound's reactivity and stability.
Recommended video:
 Verified step by step guidance
Verified step by step guidance