Here are the essential concepts you must grasp in order to answer the question correctly.
Spontaneity of Reactions
The spontaneity of a chemical reaction is determined by the change in Gibbs free energy (ΔG). A reaction is spontaneous under standard-state conditions if ΔG is negative, indicating that the products are more stable than the reactants. Factors such as enthalpy (ΔH) and entropy (ΔS) changes during the reaction also influence ΔG, as described by the equation ΔG = ΔH - TΔS.
Recommended video:
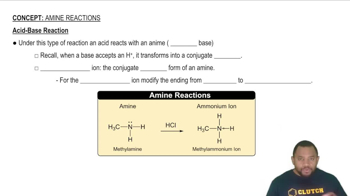
Role of Bases in Reactions
In organic reactions, bases can facilitate the removal of acidic byproducts, such as HCl in this case, which can shift the equilibrium towards product formation. By removing HCl, the reaction may proceed more favorably, potentially increasing the yield of vinyl chloride. This principle is often applied in reactions where the formation of a stable product is hindered by the presence of acidic species.
Recommended video:
Synthesis from Basic Reactants
The synthesis of vinyl chloride from graphite, H2, and Cl2 involves understanding the reactivity of these substances under specific conditions. At 25 °C and 1 atm, the reaction would require appropriate activation energy and conditions to proceed. The feasibility of this synthesis depends on the thermodynamics and kinetics of the reaction, including the formation of intermediates and the stability of the final product.
Recommended video:
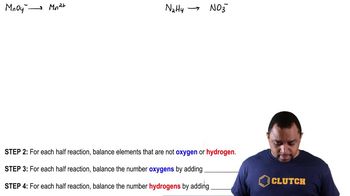
Balancing Basic Redox Reaction Example
 McMurry 8th Edition
McMurry 8th Edition Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium
Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium Problem 105
Problem 105 Verified step by step guidance
Verified step by step guidance