Here are the essential concepts you must grasp in order to answer the question correctly.
Henry's Law
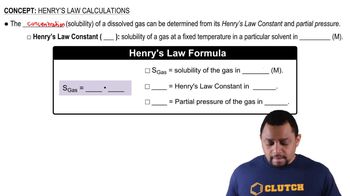
Henry's Law states that the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid. This relationship can be expressed mathematically as C = kP, where C is the concentration of the gas in the liquid, k is the Henry's law constant, and P is the partial pressure of the gas. Understanding this law is crucial for calculating the solubility of gases in liquids, such as oxygen in blood.
Recommended video:
Partial Pressure
Partial pressure is the pressure exerted by a single component of a gas mixture. In the context of the scuba diver, the total pressure at a depth of 66 ft is 3.0 atm, and the partial pressure of oxygen can be calculated by multiplying the total pressure by the fraction of oxygen in the air (21%). This concept is essential for determining how much oxygen is available for dissolution in the diver's blood.
Recommended video:
Partial Pressure Calculation
Gas Solubility in Blood
The solubility of gases in blood is influenced by factors such as temperature, pressure, and the specific gas involved. For oxygen, its solubility can be calculated using Henry's Law, taking into account the partial pressure of oxygen and the Henry's law constant for oxygen in blood. This concept is vital for understanding how oxygen is transported in the body, especially under varying pressure conditions experienced by divers.
Recommended video:
 Verified step by step guidance
Verified step by step guidance