(a) How are the boundaries between the regions of the atmosphere determined?
Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (b) How many ozone molecules are in 1.0 L of air in Mexico City? Assume T = 25 °C.

Verified Solution
Key Concepts
Ideal Gas Law

Molar Volume of a Gas

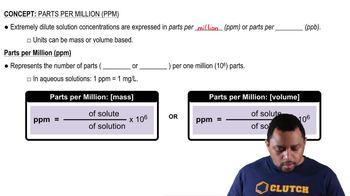
Concentration Units (ppb and ppm)
(b) Explain why the stratosphere, which is about 35 km thick, has a smaller total mass than the troposphere, which is about 12 km thick.
Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (a) Calculate the partial pressure of ozone at 441 ppb if the atmospheric pressure is 0.67 atm.
From the data in Table 18.1, calculate the partial pressures of carbon dioxide and argon when the total atmospheric pressure is 1.05 bar.
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
(b) Use the energy requirements of these two pro- cesses to explain why photodissociation of oxygen is more important than photoionization of oxygen at altitudes below about 90 km.
