Here are the essential concepts you must grasp in order to answer the question correctly.
Chlorofluorocarbons (CFCs)
Chlorofluorocarbons are organic compounds that contain chlorine, fluorine, and carbon. They were commonly used as refrigerants, propellants, and solvents. However, CFCs are known to deplete the ozone layer, leading to increased UV radiation reaching the Earth's surface. Due to their environmental impact, many CFCs have been phased out under international agreements like the Montreal Protocol.
Hydrofluorocarbons (HFCs)
Hydrofluorocarbons are a class of compounds that contain hydrogen, fluorine, and carbon. Unlike CFCs, HFCs do not contain chlorine and therefore do not deplete the ozone layer. They are often used as substitutes for CFCs in refrigeration and air conditioning. However, HFCs are potent greenhouse gases, contributing to global warming, which has led to calls for their regulation and reduction.
Environmental Impact
The environmental impact of chemical compounds like CFCs and HFCs is crucial for understanding their use and regulation. CFCs contribute to ozone layer depletion, which increases UV radiation exposure, while HFCs, although ozone-friendly, are significant greenhouse gases. Understanding these impacts is essential for evaluating the sustainability of these compounds and the need for alternatives in industrial applications.
Recommended video:
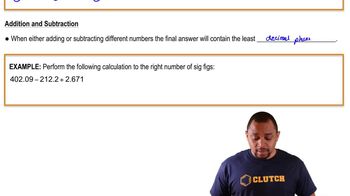
Significant Figures in Addition and Substraction