Textbook Question
Give the electron-domain and molecular geometries for the following molecules and ions: c. SF4
2
views
 Verified step by step guidance
Verified step by step guidance
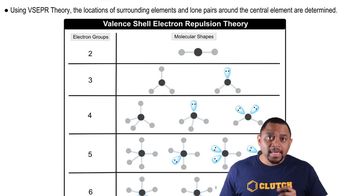

Give the electron-domain and molecular geometries for the following molecules and ions: c. SF4
Draw the Lewis structure for each of the following molecules or ions, and predict their electron-domain and molecular geometries: (e) XeF2
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (c) Which of the following elements will lead to an AF4 molecule with the shape in (iii): Be, C, S, Se, Si, Xe? i.
ii.
iii.
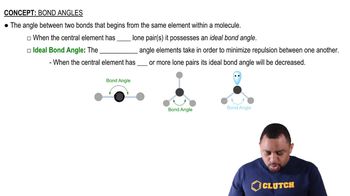
Give the approximate values for the indicated bond angles in the following molecules: (c)
Give the approximate values for the indicated bond angles in the following molecules: (a)
Give the approximate values for the indicated bond angles in the following molecules: (d)