(b) Write the chemical equation that represents the process of lattice energy for the case of NaCl.
NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (b) Use the ionic radii given in Figure 7.8 to estimate the Na─Cl and K─F distances.

Verified Solution
Key Concepts
Ionic Radii
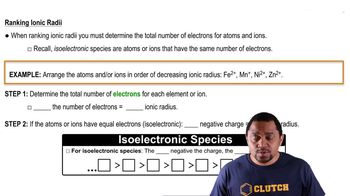
Crystal Structure

Ionic Bonding

(c) Would you expect salts like NaCl, which have singly charged ions, to have larger or smaller lattice energies compared to salts like CaO which are composed of doubly-charged ions?
NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (a) The lattice energies of NaCl and KF are given in Table 8.1. Based on the lattice energies, would you expect the Na─Cl or the K─F distance to be longer?
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (a) What are the charges on each of the cations in each compound?
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (b) What are the charges of each of the anions in each compound?
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (d) Using the lattice energies in Table 8.1, predict the lattice energy of ScN.
