Textbook Question
Identify each statement as true or false: (b) Li+ is smaller than Li.
371
views
 Verified step by step guidance
Verified step by step guidance
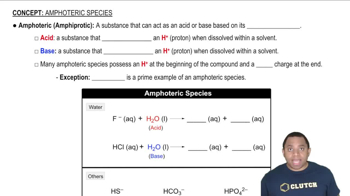


Identify each statement as true or false: (b) Li+ is smaller than Li.
For each set of atoms and ions, pick the smallest one.
a. I, I+, I−
b. Be2+, Ca2+, Mg2+
c. Fe, Fe2+, Fe3+
Which neutral atom is isoelectronic with each of the following ions? Ga3+, Zr4+, Mn7+, I−, Pb2+.
Consider the isoelectronic ions F- and Na+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions.
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.