Textbook Question
For each set of atoms and ions, pick the smallest one.
a. I, I+, I−
b. Be2+, Ca2+, Mg2+
c. Fe, Fe2+, Fe3+
 Verified step by step guidance
Verified step by step guidance
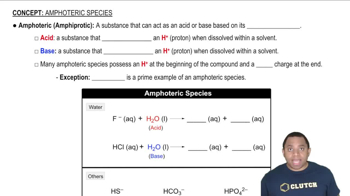
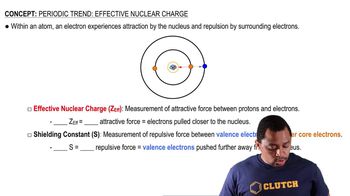

For each set of atoms and ions, pick the smallest one.
a. I, I+, I−
b. Be2+, Ca2+, Mg2+
c. Fe, Fe2+, Fe3+
Which neutral atom is isoelectronic with each of the following ions? Ga3+, Zr4+, Mn7+, I−, Pb2+.
For each ion, identify the neutral atom that is isoelectronic with it.
a. Cl−
b. Sc3+
c. Fe2+
d. Zn2+
e.Sn4+
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.
Consider S, Cl, and K and their most common ions.(c) Explain any differences in the orders of the atomic and ionic sizes.