(b) A particular chip snack food is composed of 12% protein, 14% fat, and the rest carbohydrate. What percentage of the calorie content of this food is fat?
The heat of combustion of ethanol, C2H5OH(l), is -1367 kJ/mol. A batch of Sauvignon Blanc wine contains 10.6% ethanol by mass. Assuming the density of the wine to be 1.0 g/mL, what is the caloric content due to the alcohol (ethanol) in a 6-oz glass of wine (177 mL)?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Heat of Combustion

Mass Percent Concentration

Density and Volume Conversion
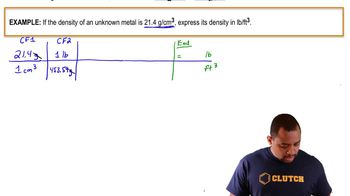
(a) A serving of a particular ready-to-serve chicken noodle soup contains 2.5 g fat, 14 g carbohydrate, and 7 g protein. Estimate the number of Calories in a serving.
The heat of combustion of fructose, C6H12O6, is -2812 kJ/mol. If a fresh golden delicious apple weighing 4.23 oz (120 g) contains 16.0 g of fructose, what caloric content does the fructose contribute to the apple?
The standard enthalpies of formation of gaseous propyne (C3H4), propylene (C3H6), and propane (C3H8) are +185.4, +20.4, and -103.8 kJ/mol, respectively. (b) Calculate the heat evolved on combustion of 1 kg of each substance.
The standard enthalpies of formation of gaseous propyne (C3H4), propylene (C3H6), and propane (C3H8) are +185.4, +20.4, and -103.8 kJ/mol, respectively. (c) Which is the most efficient fuel in terms of heat evolved per unit mass?
At the end of 2020, global population was about 7.8 billion. What mass of glucose in kg would be needed to provide 1500 Cal/person/day of nourishment to the global population for one year? Assume that glucose is metabolized entirely to CO2(𝑔) and H2O(𝑙) according to the following thermochemical equation: C6H12O6(s) + 6 O2(𝑔) → 6 CO2(𝑔) + 6 H2O(𝑙) ΔH° = -2803 kJ
