Calculate the percentage by mass of the indicated element in the following compounds: d. platinum in PtCl2(NH3)2, a chemotherapy agent called cisplatin
Ch.3 - Chemical Reactions and Reaction Stoichiometry
Chapter 3, Problem 27
Based on the following structural formulas, calculate the percentage of carbon by mass present in each compound: (a) Benzaldehyde (almond fragrance) (b) Vanillin (vanilla flavor) c) Isopentyl acetate (banana flavor)
 Verified step by step guidance
Verified step by step guidance1
1. First, you need to know the molecular formula of each compound. The molecular formula of Benzaldehyde is C7H6O, Vanillin is C8H8O3, and Isopentyl acetate is C7H14O2.
2. Next, calculate the molar mass of each compound. The molar mass is calculated by adding up the atomic masses of each element in the compound, multiplied by the number of atoms of that element in the compound. For example, the molar mass of Benzaldehyde is (7*12.01 g/mol for C) + (6*1.008 g/mol for H) + (1*16.00 g/mol for O).
3. Then, calculate the total mass of carbon in each compound. This is done by multiplying the number of carbon atoms in the compound by the atomic mass of carbon (12.01 g/mol). For example, the total mass of carbon in Benzaldehyde is 7*12.01 g/mol.
4. After that, calculate the percentage of carbon by mass in each compound. This is done by dividing the total mass of carbon in the compound by the molar mass of the compound, and then multiplying by 100%. For example, the percentage of carbon by mass in Benzaldehyde is (total mass of carbon / molar mass of Benzaldehyde) * 100%.
5. Repeat steps 2-4 for Vanillin and Isopentyl acetate.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass Calculation
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To calculate the molar mass of a compound, sum the atomic masses of all the atoms in its molecular formula. This is essential for determining the mass percentage of an element, as it provides the total mass needed for calculations.
Recommended video:
Guided course

Molar Mass Calculation Example
Mass Percentage
Mass percentage is a way of expressing the concentration of an element in a compound, calculated as the mass of the element divided by the total mass of the compound, multiplied by 100. This concept is crucial for understanding how much of a compound's mass is contributed by a specific element, such as carbon in the given compounds.
Recommended video:
Guided course

Mass Percent Calculation
Structural Formulas
Structural formulas represent the arrangement of atoms within a molecule, showing how atoms are bonded together. Understanding structural formulas is vital for identifying the number of carbon atoms in each compound, which directly impacts the calculation of carbon's mass percentage in the overall molecular structure.
Recommended video:
Guided course
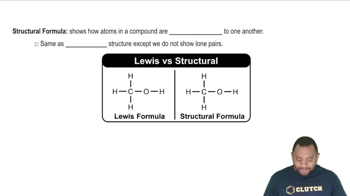
Structural Formula
Related Practice
Textbook Question
3
views
Textbook Question
Calculate the percentage by mass of the indicated element in the following compounds: e. oxygen in the female sex hormone estradiol, C18H24O2
2
views
Textbook Question
Calculate the percentage by mass of the indicated element in the following compounds: f. carbon in capsaicin, C18H27NO3, the compound that gives the hot taste to chili peppers.
2
views
Textbook Question
Calculate the percentage of carbon by mass in each of the compounds represented by the following models: (a)
395
views
Textbook Question
(a) Write 'true' or 'false' for each statement. (a) A mole of horses contain a mole of horse legs.
959
views
Textbook Question
(a) Write 'true' or 'false' for each statement. (b) A mole of water has a mass of 18.0 g.
669
views
