A voltaic cell is constructed that uses the following half-cell reactions: Cu+1aq2 + e- ¡ Cu1s2 I21s2 + 2 e- ¡ 2 I-1aq2 The cell is operated at 298 K with 3Cu+4 = 0.25 M and 3I-4 = 0.035 M. (a) Determine E for the cell at these concentrations.
Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (c) If you react two thiols to make a disulfide, are you oxidizing or reducing the thiols?

Verified Solution
Key Concepts
Oxidation and Reduction

Thiols and Disulfides
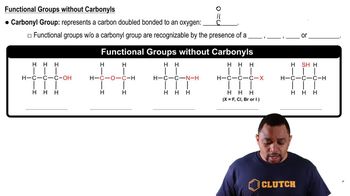
Redox Reaction in Thiol to Disulfide Conversion

The capacity of batteries such as the typical AA alkaline battery is expressed in units of milliamp-hours (mAh). An AA alkaline battery yields a nominal capacity of 2850 mAh. (b) The starting voltage of a fresh alkaline battery is 1.55 V. The voltage decreases during discharge and is 0.80 V when the battery has delivered its rated capacity. If we assume that the voltage declines linearly as current is withdrawn, estimate the total maximum electrical work the battery could perform during discharge.
Disulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (b) What is the oxidation state of sulfur in a disulfide?
Calculate the number of kilowatt-hours of electricity required to produce 1.0 * 103 kg (1 metric ton) of aluminum by electrolysis of Al3+ if the applied voltage is 4.50 V and the process is 45% efficient.
Aqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: 'Never mix ammonia and bleach, as toxic gases may be produced.' One of the toxic gases that can be produced is chloroamine, NH2Cl. (b) What is the oxidation number of chlorine in chloramine?
Aqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: 'Never mix ammonia and bleach, as toxic gases may be produced.' One of the toxic gases that can be produced is chloroamine, NH2Cl. (e) Is N oxidized, reduced, or neither, upon the conversion of ammonia to nitrogen trichloride?
