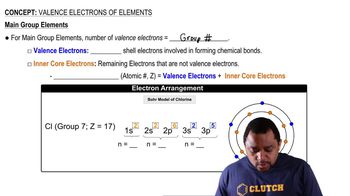
Textbook Question
A charged particle moves between two electrically charged plates, as shown here.
(a) What is the sign of the electrical charge on the particle?
686
views


 Verified step by step guidance
Verified step by step guidance


A charged particle moves between two electrically charged plates, as shown here.
(a) What is the sign of the electrical charge on the particle?
The following diagram is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293Nv, and the blue spheres are 295Nv. (b) If the mass of 293Nv is 293.15 u and that of 295Nv is 295.15 u, what is the atomic weight of Nv?
Four of the boxes in the following periodic table are colored. Which of these are metals and which are nonmetals?
Does the following drawing represent a neutral atom or an ion?