An unknown particle is caused to move between two electrically charged plates, as illustrated in Figure 2.7. You hypothesize that the particle is a proton. (a) If your hypothesis is correct, would the particle be deflected in the same or opposite direction as the b rays?
Millikan determined the charge on the electron by studying the static charges on oil drops falling in an electric field (Figure 2.5). A student carried out this experiment using several oil drops for her measurements and calculated the charges on the drops. She obtained the following data: Droplet Calculated Charge (C) A 1.60 * 10-19 B 3.15 * 10-19 C 4.81 * 10-19 D 6.31 * 10-19 (b) What conclusion can the student draw from these data regarding the charge of the electron?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Millikan's Oil Drop Experiment
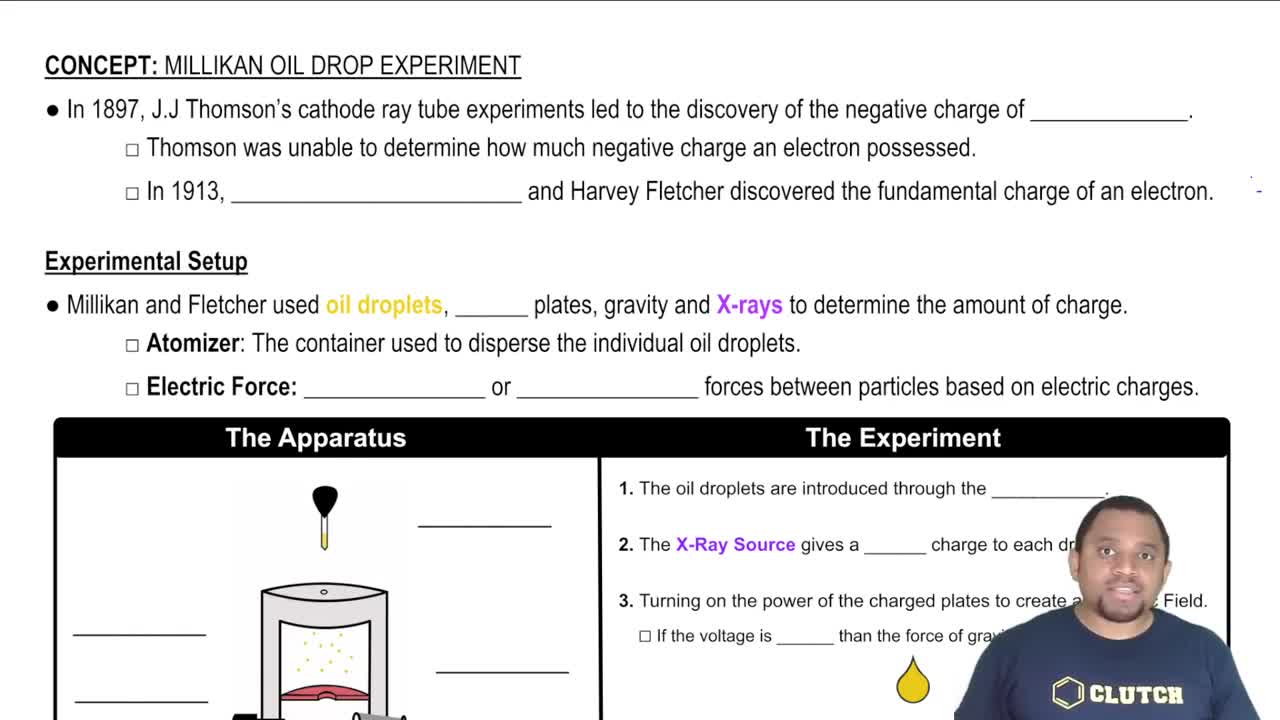
Quantization of Charge

Data Analysis and Conclusion Drawing

An unknown particle is caused to move between two electrically charged plates, as illustrated in Figure 2.7. You hypothesize that the particle is a proton. (b) Would it be deflected by a smaller or larger amount than the b rays?
Which set of statements is true about Rutherford’s gold foil experiment?
i. This is the main experiment that showed that atoms have a dense nucleus.
ii. The data from the experiment showed that alpha particles scattered equally at all angles from the gold foil.
iii. Electrons were emitted from the gold atoms in straight lines.
a. i only
b. ii only
c. iii only
d. i and ii
e. i and iii
Millikan determined the charge on the electron by studying the static charges on oil drops falling in an electric field (Figure 2.5). A student carried out this experiment using several oil drops for her measurements and calculated the charges on the drops. She obtained the following data: Droplet Calculated Charge (C) A 1.60 * 10-19 B 3.15 * 10-19 C 4.81 * 10-19 D 6.31 * 10-19 (c) What value (and to how many significant figures) should she report for the electronic charge?
The radius of an atom of gold (Au) is about 1.35 Å. a. Express this distance in nanometers (nm) and in picometers (pm).
The radius of an atom of gold (Au) is about 1.35 Å. b. How many gold atoms would have to be lined up to span 1.0 mm?
