Here are the essential concepts you must grasp in order to answer the question correctly.
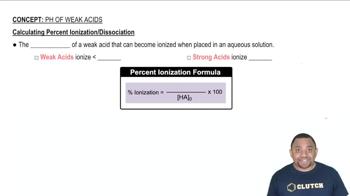
Ionization of Weak Acids
Weak acids, like bromoacetic acid, do not fully dissociate in solution. The degree of ionization indicates how much of the acid has converted to its ions, which is crucial for calculating concentrations of the species in solution. In this case, a 13.2% ionization means that 13.2% of the bromoacetic acid molecules have dissociated into H+ and BrCH2COO- ions.
Recommended video:
Calculating Percent Ionization of Weak Acids
Equilibrium Constant (Ka)
The acid dissociation constant (Ka) quantifies the strength of a weak acid in solution. It is calculated using the concentrations of the products and reactants at equilibrium. For bromoacetic acid, Ka can be determined from the concentrations of H+, BrCH2COO-, and BrCH2COOH at equilibrium, reflecting the extent of ionization.
Recommended video:
Concentration Calculations
To find the concentrations of H+, BrCH2COO-, and BrCH2COOH, one must apply the initial concentration and the degree of ionization. For a 0.100 M solution that is 13.2% ionized, the concentration of H+ and BrCH2COO- can be calculated as 0.0132 M, while the remaining concentration of BrCH2COOH can be found by subtracting the ionized amount from the initial concentration.
Recommended video:
Calculate Concentration of the Basic Form