Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
When ammonium chloride dissolves in water, the solution becomes colder. (b) Why does the solution form?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Dissolution Process

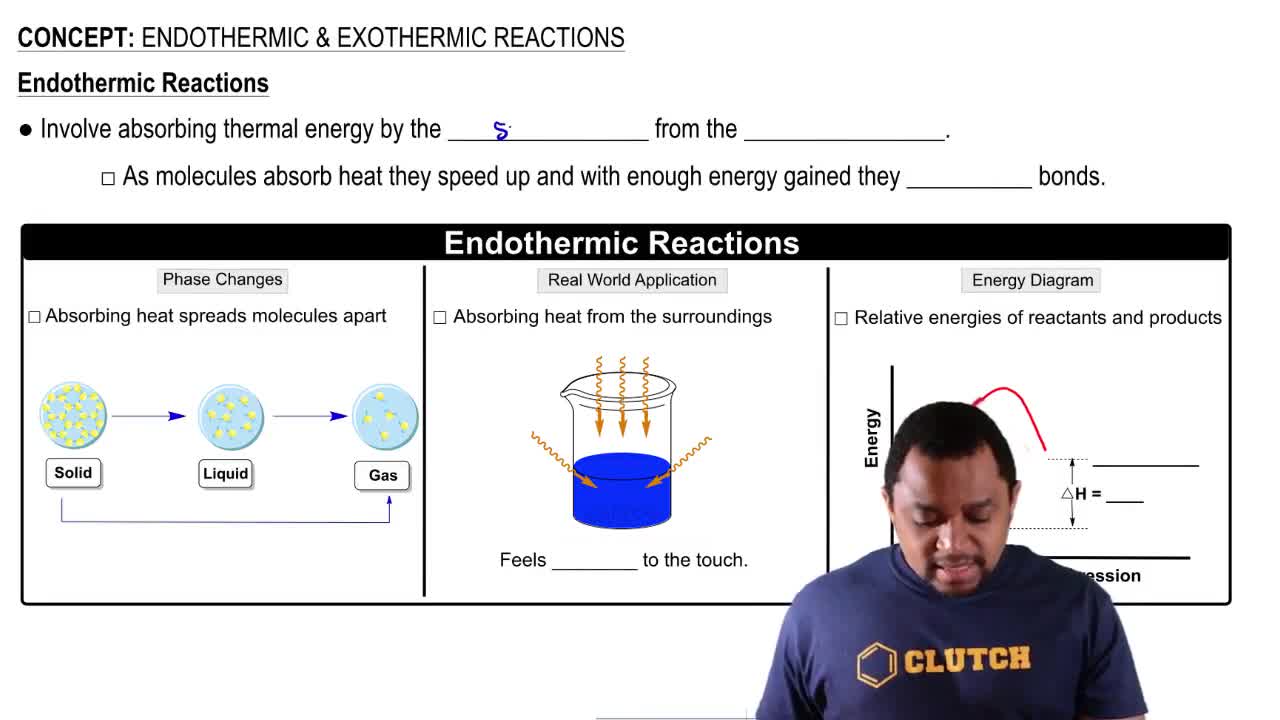
Endothermic Reactions
Thermodynamics of Solutions

An ionic compound has a very negative ∆Hsoln in water (b) Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?
When ammonium chloride dissolves in water, the solution becomes colder. (a) Is the solution process exothermic or endothermic?
Two nonpolar organic liquids, hexane (C6H14) and heptane (C7H16), are mixed. (a) Do you expect ∆Hsoln to be a large positive number, a large negative number, or close to zero? Explain.
Two nonpolar organic liquids, hexane (C6H14) and heptane (C7H16), are mixed. (b) Hexane and heptane are miscible with each other in all proportions. In making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure liquids?
KBr is relatively soluble in water, yet its enthalpy of solution is + 19.8 kJ/mol. Which of the following statements provides the best explanation for this behavior? (a) Potassium salts are always soluble in water. (b) The entropy of mixing must be unfavorable. (c) The enthalpy of mixing must be small compared to the enthalpies for breaking up water–water interactions and K–Br ionic interactions. (d) KBr has a high molar mass compared to other salts like NaCl.
