By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (d) Pb(NO3)2.
Ch.13 - Properties of Solutions
Chapter 13, Problem 28
Oil and water are immiscible. Which is the most likely reason? (a) Oil molecules are denser than water. (b) Oil molecules are composed mostly of carbon and hydrogen. (c) Oil molecules have higher molar masses than water. (d) Oil molecules have higher vapor pressures than water. (e) Oil molecules have higher boiling points than water.
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of immiscibility: Immiscibility refers to the inability of two substances to mix and form a homogeneous solution. In the case of oil and water, they do not mix because of differences in their molecular properties.
Consider the polarity of molecules: Water is a polar molecule, meaning it has a partial positive charge on one side and a partial negative charge on the other. Oil, on the other hand, is nonpolar, primarily composed of carbon and hydrogen atoms, which do not have significant charge separation.
Apply the 'like dissolves like' principle: This principle states that polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents. Since oil is nonpolar and water is polar, they do not mix well.
Evaluate the options: (a) Density, (c) molar mass, (d) vapor pressure, and (e) boiling point are not directly related to the immiscibility of oil and water. The key factor is the molecular composition and polarity, which is addressed in option (b).
Conclude that the most likely reason for the immiscibility of oil and water is option (b): Oil molecules are composed mostly of carbon and hydrogen, making them nonpolar and unable to mix with polar water molecules.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity
Polarity refers to the distribution of electrical charge over the atoms in a molecule. Water is a polar molecule, meaning it has a partial positive charge on one side and a partial negative charge on the other, allowing it to form hydrogen bonds. In contrast, oil molecules are nonpolar, lacking significant charge separation, which prevents them from interacting favorably with water molecules, leading to immiscibility.
Recommended video:
Guided course

Molecular Polarity
Hydrophobic and Hydrophilic Interactions
Hydrophobic interactions occur between nonpolar substances, like oil, which do not mix well with polar substances, such as water. Hydrophilic substances, on the other hand, are attracted to water. The inability of oil to form hydrogen bonds with water molecules results in the separation of the two liquids, as oil molecules prefer to associate with each other rather than with water.
Recommended video:
Guided course
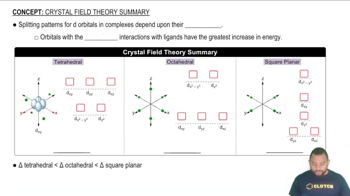
The greatest ligand-orbital interactions result in the greatest increase in energy.
Density and Miscibility
Density is a measure of mass per unit volume and can influence the behavior of liquids. While oil is generally less dense than water, which causes it to float, the primary reason for their immiscibility is not density but rather the differences in polarity. Miscibility is determined by the ability of substances to mix at a molecular level, which is hindered in this case due to the nonpolar nature of oil.
Recommended video:
Guided course

Density Concepts
Related Practice
Textbook Question
875
views
Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
436
views
Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
967
views
2
rank
Textbook Question
Which of the following in each pair is likely to be more soluble in hexane, C6H14: b. benzene (C6H6) or glycerol, CH2(OH)CH(OH)CH2OH,
2190
views
Textbook Question
Which of the following in each pair is likely to be more soluble in water: (a) cyclohexane (C6H12) or glucose (C6H12O6),
1027
views
Textbook Question
Which of the following in each pair is likely to be more soluble in water: (c) HCl or ethyl chloride (CH3CH2Cl)? Explain in each case.
501
views
