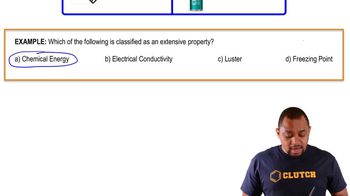
Shown here are sketches of two processes. Which of the processes refers to the ductility of metals and which refers to malleability of metals? (a)
(b)

 Verified step by step guidance
Verified step by step guidance
Shown here are sketches of two processes. Which of the processes refers to the ductility of metals and which refers to malleability of metals? (a)
(b)
(b) What is the coordination number of each cannonball in the interior of the stack?
Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your reasoning. (a)
(b)
The electronic structure of a doped semiconductor is shown here. (c) Which region of the diagram represents the band gap?
Shown here are cartoons of two different polymers. Which one would have the higher melting point?
The accompanying image shows photoluminescence from four different samples of CdTe nanocrystals, each embedded in a polymer matrix. The photoluminescence occurs because the samples are being irradiated by a UV light source. The nanocrystals in each vial have different average sizes. The sizes are 4.0, 3.5, 3.2, and 2.8 nm. (a) Which vial contains the 4.0-nm nanocrystals?