Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your reasoning. (a)
(b)

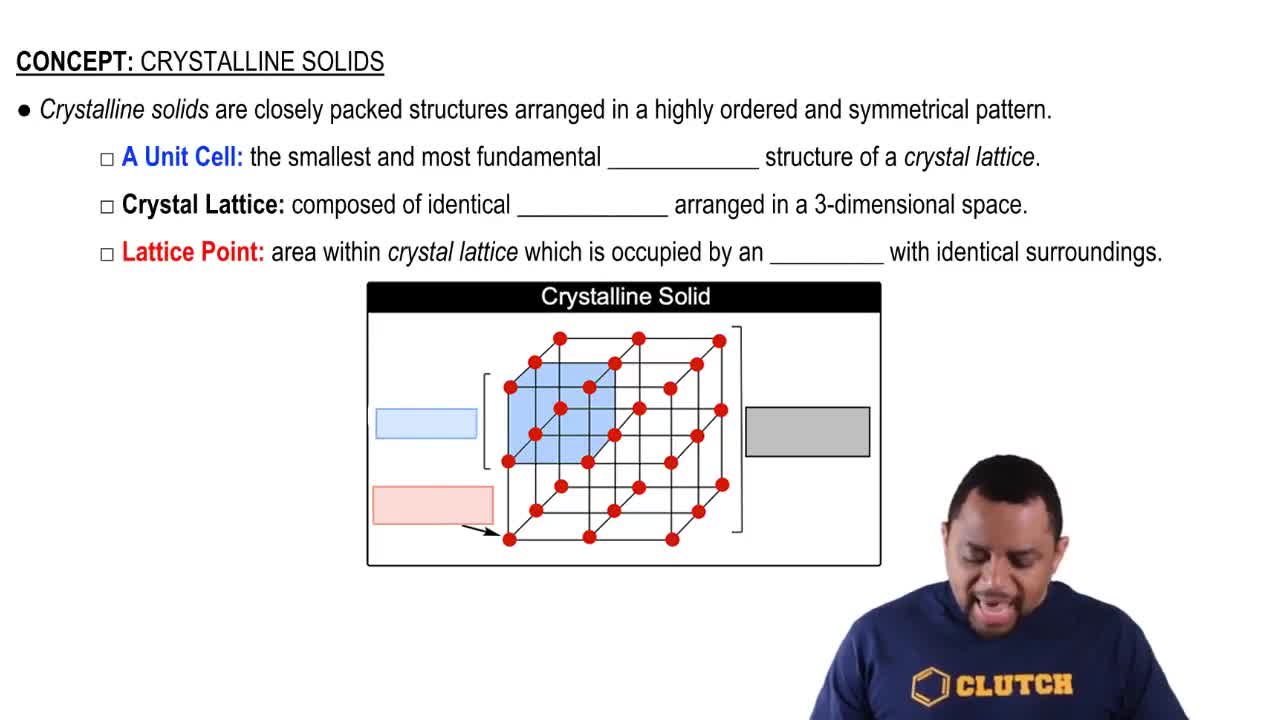
 Verified step by step guidance
Verified step by step guidance


Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your reasoning. (a)
(b)
Which of these molecular fragments would you expect to be more likely to give rise to electrical conductivity? Explain your reasoning. (a)
(b)
The electronic structure of a doped semiconductor is shown here. (c) Which region of the diagram represents the band gap?
The accompanying image shows photoluminescence from four different samples of CdTe nanocrystals, each embedded in a polymer matrix. The photoluminescence occurs because the samples are being irradiated by a UV light source. The nanocrystals in each vial have different average sizes. The sizes are 4.0, 3.5, 3.2, and 2.8 nm. (a) Which vial contains the 4.0-nm nanocrystals?
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (a) Is Si a molecular, metallic, ionic, or covalent-network solid?
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (b) Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid?