The rate of solar energy striking Earth averages 168 watts per square meter. The rate of energy radiated from Earth's surface averages 390 watts per square meter. Comparing these numbers, one might expect that the planet would cool quickly, yet it does not. Why not?
Write balanced chemical equations for each of the following reactions: (d) Nitrogen dioxide dissolves in water to form nitric acid and nitric oxide.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
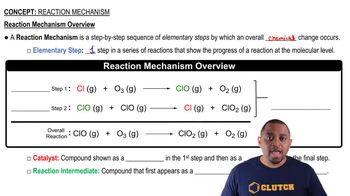
Key Concepts
Chemical Equations

Acid-Base Reactions

Dissolution and Reaction Mechanisms
The solar power striking Earth every day averages 168 watts per square meter. The highest ever recorded electrical power usage in New York City was 13,200 MW. A record established in July of 2013. Considering that present technology for solar energy conversion is about 10% efficient, from how many square meters of land must sunlight be collected in order to provide this peak power? (For compar- ison, the total area of New York City is 830 km2.)
Write balanced chemical equations for each of the following reactions: (b) The nitric oxide molecule undergoes photoionization in the upper atmosphere.
(b) Will Mg(OH)2 precipitate when 4.0 g of Na2CO3 is added to 1.00 L of a solution containing 125 ppm of Mg2+?
(b) Concentrations of lead in the bloodstream are often quoted in units of μg/dL. Averaged over the entire country, the mean concentration of lead in the blood was measured to be 1.6 μg/dL in 2008. Express this concentration in ppb.
