Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reac- tion is 335 nm. (d) Write out the formaldehyde photodis- sociation reaction, showing Lewis-dot structures.
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. Assuming that the phosphorus is present as dihydrogenphosphate, H2PO4-, calculate the correspond-ing molar concentration of H2PO4- in seawater.

Verified Solution
Key Concepts
Parts Per Million (ppm)
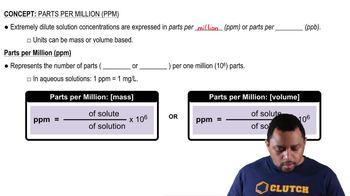
Molar Concentration

Molar Mass

An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reac- tion is 420 nm. (a) In what part of the electromagnetic spec- trum is light with this wavelength found?
What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g>mL?
The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that evaporation of water is due only to energy input from the Sun, calculate how many grams of water could be evaporated from a 1.00 square meter patch of ocean over a 12-h day
The enthalpy of fusion of water is 6.01 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (b) The specific heat capacity of ice is 2.032 J/g°C. If the initial temperature of a 1.00 square emter patch of ice is -5.0°C, what is its final temperature after being in sunlight for 12 h, assuming no phase changes and assuming that sunlight penetration uniformly to a depth of 1.00 cm?
The Ogallala aquifer described in the Closer Look box in Section 18.3, provides 82% of the drinking water for the people who live in the region, although more than 75% of the water that is pumped from it is for irrigation. Irrigation withdrawals are approximately 18 billion gallons per day. (a) Assuming that 2% of the rainfall that falls on an area of 600,000 km2 recharges the aquifer, what average annual rainfall would be required to replace the water removed for irrigation?
