Textbook Question
(a) How are the boundaries between the regions of the atmosphere determined?
322
views
 Verified step by step guidance
Verified step by step guidance


(a) How are the boundaries between the regions of the atmosphere determined?
(b) Explain why the stratosphere, which is about 35 km thick, has a smaller total mass than the troposphere, which is about 12 km thick.
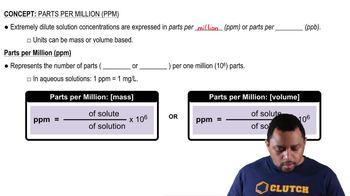
Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (a) Calculate the partial pressure of ozone at 441 ppb if the atmospheric pressure is 0.67 atm.
From the data in Table 18.1, calculate the partial pressures of carbon dioxide and argon when the total atmospheric pressure is 1.05 bar.