An AB2 molecule is described as having a tetrahedral geometry. (b) Based on the information given, which of the following is the molecular geometry of the molecule: (i) linear, (ii) bent, (iii) trigonal planar, or (iv) tetrahedral?
Consider the molecule PF4Cl. (c) Predict the molecular geometry of PF4Cl. How did your answer for part (b) influence your answer here in part (c)?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
VSEPR Theory
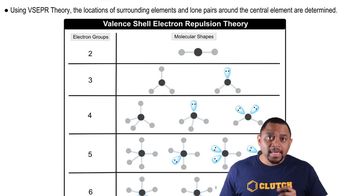
Molecular Geometry

Influence of Hybridization

Consider the following XF4 ions: PF4-, BrF4-, ClF4+, and AlF4-. (a) Which of the ions have more than an octet of electrons around the central atom?
Consider the following XF4 ions: PF4-, BrF4-, ClF4+, and AlF4-. (c) Which of the ions will have an octahedral electron-domain geometry?
Consider the molecule PF4Cl. (d) Would you expect the molecule to distort from its ideal electron-domain geometry? If so, how would it distort?
Fill in the blank spaces in the following chart. If the molecule column is blank, find an example that fulfills the conditions of the rest of the row. Molecule Electron-Domain Hybridization Dipole Geometry of Central Atom Moment? Yes or No CO2 sp3 Yes sp3 No Trigonal planar No SF4 Octahedral No sp2 Yes Trigonal bipyramidal No XeF2 Complete the first row of the table.
